- adapted from: Thomas Bibby, Jon Nield, James Barber and colleagues:
Nature: 412:743-745, Nature 413:590 and Nature 424:1051-1054.
Although iron is the fourth most abundant element in the Earth's crust, its concentration, particularly the open oceans, is sufficiently low to limit photosynthetic activity and phytoplankton growth.
Cyanobacteria, a major class of phytoplankton, respond to iron deficiency by expressing the `iron-stress-induced' gene, isiA. The protein encoded by this gene, called CP43’ (or isiA) has an amino-acid sequence that shows significant homology with one of the chlorophyll a-binding proteins (CP43) of photosystem II (PSII).
CP43' associates with photosystem I (PSI) to form a complex that consists of a ring of 18 CP43' molecules around a-PSI trimer. This significantly increases the size of the light-harvesting system of-PSI. The utilisation of a PSII-like protein as an extra antenna for PSI emphasises the flexibility of cyanobacterial light-harvesting systems and seems to be a strategy which compensates for the lowering of phycobilisome and PSI levels in response to iron deficiency.
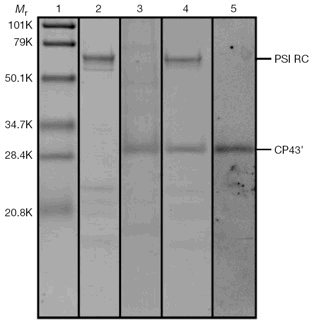
Fig. 1. SDS-PAGE of fractions derived from sucrose density centrifugation.
Of note: lane 3, CP43'; lane 4, CP43'-PSI complex.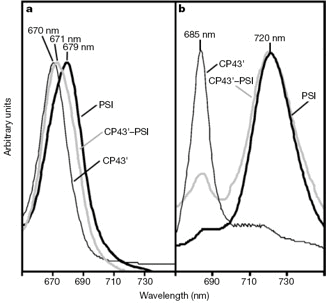
Fig. 2. Optical absorption and fluorescence properties of sucrose density centrifugation fractions.
a, Room-temp. absorption spectra. b, Fluorescence emission spectra, 77 K excited w/440 nm light.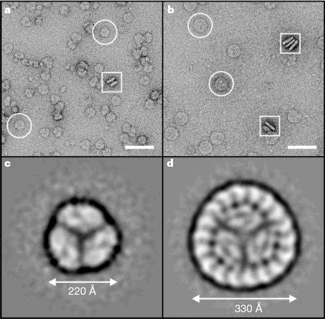
Fig. 3. Electron micrographs and processed top views of the PSI trimer and CP43-PSI supercomplex.
PSI trimers (a) and CP43'-PSI complex (b) in negative stain; scale bar, 50 nm.
Image-processed top views of negatively stained-PSI trimers (c) and CP43’-PSI complexes (d).
The projection maps were obtained by averaging 4,000-PSI trimer and 3,000 CP43'-PSI particles.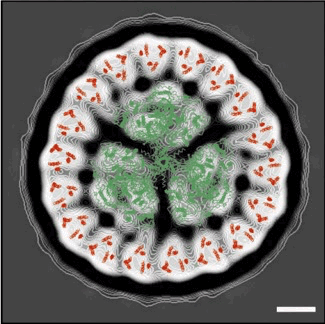
Fig. 4. The-PSI trimer structure and CP43 helix organisation derived from X-ray crystallography (elsewhere) overlaid onto the projection map of the CP43'-PSI complex. Scale bar, 5 nm. Good correlation between the X-ray and electron microscopy data confirms the presence of a-PSI trimer within the centre of an 18-member ring of CP43’.√
© Nature, Macmillan Magazines Ltd, 2001
In 3D with negative stain:
(Thomas S. Bibby, Jon Nield & James Barber. J. Biol. Chem. 2001):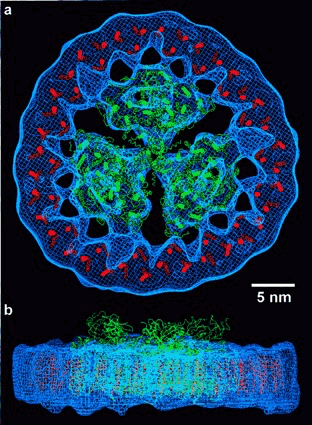
Fig. 7. Modeling of coordinate data sets within the calculated three-dimensional map. Top view (a) and side view (b) showing the three-dimensional map as chicken wire in blue, modelled carbon-alpha trace of the PSI trimer built from coordinate data set IC51 in green, and the 6-transmembrane helices of CP43 from the PSII structure (28) IFE1 in red. Bar represents 5 nm.
© Journal of Biological Chemistry, 2001
Then, in 3D, under cryo-conditions, no stain:
(Jon Nield, Edward Morris, Thomas Bibby & James Barber. Biochemistry 2003):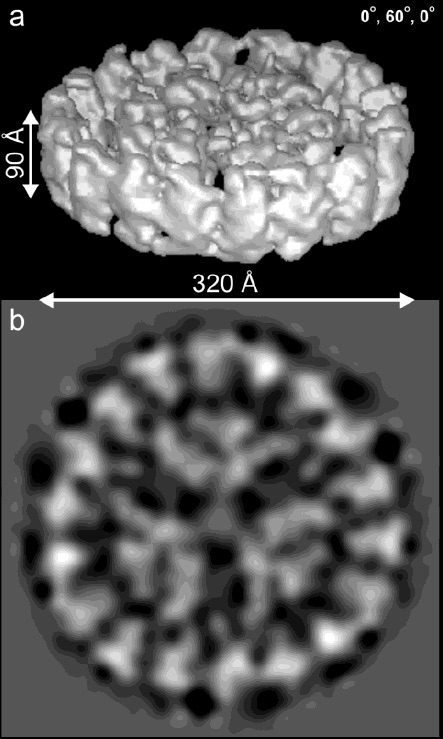
Cryo-EM map (above) of the CP43'-PSI supercomplex at ~ 20 Å resolution.
(a) Surface representation of the 3D structure shown at an oblique angle.
(b) Top view 2D projection map.
© Biochemistry, 2003